Product
Biomembretics is more than just a product…it is an entirely new frontier: a biomimetic (“mimics biology”) membrane technology platform that blends nature’s design for human organs into existing artificial organ machine devices. Developed in collaboration with Draper and the U. S. Department of Defense, these bio-membranes incorporate a proprietary computational process and patented 3D, microfluidic design into compact disposable membrane filters.
Our first product will be a microfluidic bio-membrane that replaces the artificially structured filter currently used in artificial lung machines (known as extracorporeal membrane oxygenation or “ECMO”). These bio-membranes will seamlessly integrate into ECMO devices to transform the safety, efficacy, and versatility of this potentially life-saving therapy.


Mission
At Biomembretics, our interdisciplinary teamwork and collaborations are developing the optimal blend of nature and machine to transform the treatment of patients with lung failure.
Dr. Todd Astor, the company Founder, is a physician who specializes in lung failure and lung transplantation. For more than 20 years, and especially throughout the COVID19 pandemic, he has experienced the heartache and frustration of watching so many patients with lung failure die because of insufficient technology to support a patient’s failing lungs.
He launched Biomembretics with the mission to bring new hope for patients through the realization of a pioneering frontier of artificial organ technology; beginning with a novel approach that mimics the physiologic blood flow and safe, effective filtering performance of the human lungs.
Technology
Value Proposition
The biomimetic design of the microfluidic bio-membrane reduces blood damage and clotting, and improves the device’s filtering efficiency for oxygen and carbon dioxide. These advantages will decrease complications and improve survival for patients with lung failure; reduce the complexity, monitoring, and complication-related costs for hospitals; and significantly increase the number of patients treated with ECMO therapy. The patented fabrication processes and platform technology capabilities will also create significant value for industry partners to transform current and future lung failure devices.

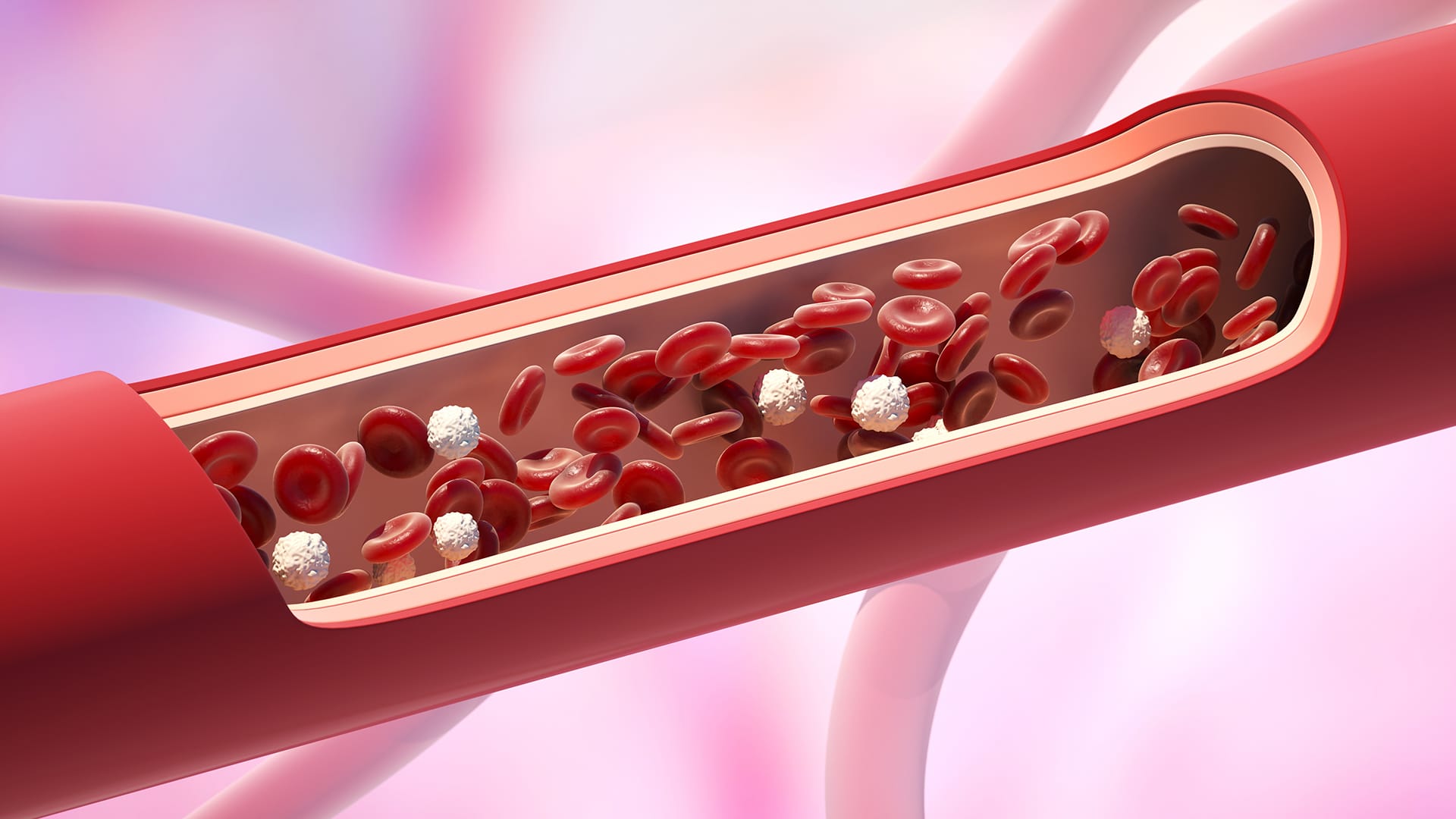
How it Works
Our lungs work as essential gas filters – filtering oxygen into the blood, and carbon dioxide out. When the lungs completely fail, we can use ECMO, a blood-filtering device, to replace this function. But the artificial design of the device filters leads to unnatural blood flow, causing blood clotting and damage.
The 3D, microfluidic channel structure and branching pattern of the bio-membranes precisely mimic the blood circuitry and physics of blood flow in the lung, eliminating the non-uniform, high shear, and high pressure flow characteristics of current filters. This natural flow creates a safe environment for the blood to eliminate blood clotting and damage, improving the filtering efficiency and efficacy of the device.
Use Cases
Lung Failure
Every year millions of patients are admitted to intensive care units (ICUs) for the treatment of lung failure. But mechanical ventilators are often ineffective, and can inflict additional damage to the lungs. ECMO is currently used to treat 10-15,000 patients per year, but the widespread use of this therapy has been impeded by complications related to blood clotting and damage that occur primarily in the device filters.
The advanced capabilities and hemocompatibility of our bio-inspired microfluidic lung membrane will:
- Improve outcomes and costs to increase the use of ECMO therapy, with a potential to drive the ECMO market to $30B.
- Expand ECMO treatment for lung failure related to a variety of advanced lung diseases, including the acute respiratory distress syndrome (ARDS), viral infections (such as COVID19 and influenza), COPD, cystic fibrosis, and pulmonary fibrosis.
- Eliminate blood clotting and the need for blood thinner medications, allowing ECMO to be used for patients with high bleeding risks, including civilian and military trauma victims.

Kidney Failure
More than 2.3M people in the U.S. currently undergo hemodialysis (HD) for kidney failure, and the market for HD is projected to reach $100B in 2025.
A microfluidic kidney bio-membrane would:
- Integrate into standard dialysis machines, greatly enhancing the filtering capabilities and hemocompatability associated with acute and chronic dialysis therapies.
- Lead to significant improvements in patient HD outcomes and costs, and help accelerate the evolution of HD from hospitals and dialysis centers to in-home use.
Other Applications
Additional applications related to other types of organ failure and immune system regulation are currently being investigated at various stages of R&D.
Partners
Biomembretics is collaborating with Draper in the development of the microfluidic membrane technology, and benefits from valuable partnerships and support from the FEDTECH Startup Studio, MassMEDIC Ignite Accelerator, and Octane Launchpad SBDC.